The Rop (Repressor of Primer) protein is a small, homodimeric protein found in bacteria, particularly in Escherichia coli. It plays a crucial role in the regulation of plasmid replication, specifically in the control of copy number for certain plasmids, such as ColE1-type plasmids. It does so by promoting the conversion of the unstable RNA I – RNA II complex to a stable complex which directly decreases copy number. It is a key component especially of the pBR322 plasmid although the original source is the pMB1 plasmid.
Understanding the structure and function of the Rop protein provides insight into bacterial plasmid maintenance and genetic regulation mechanisms.
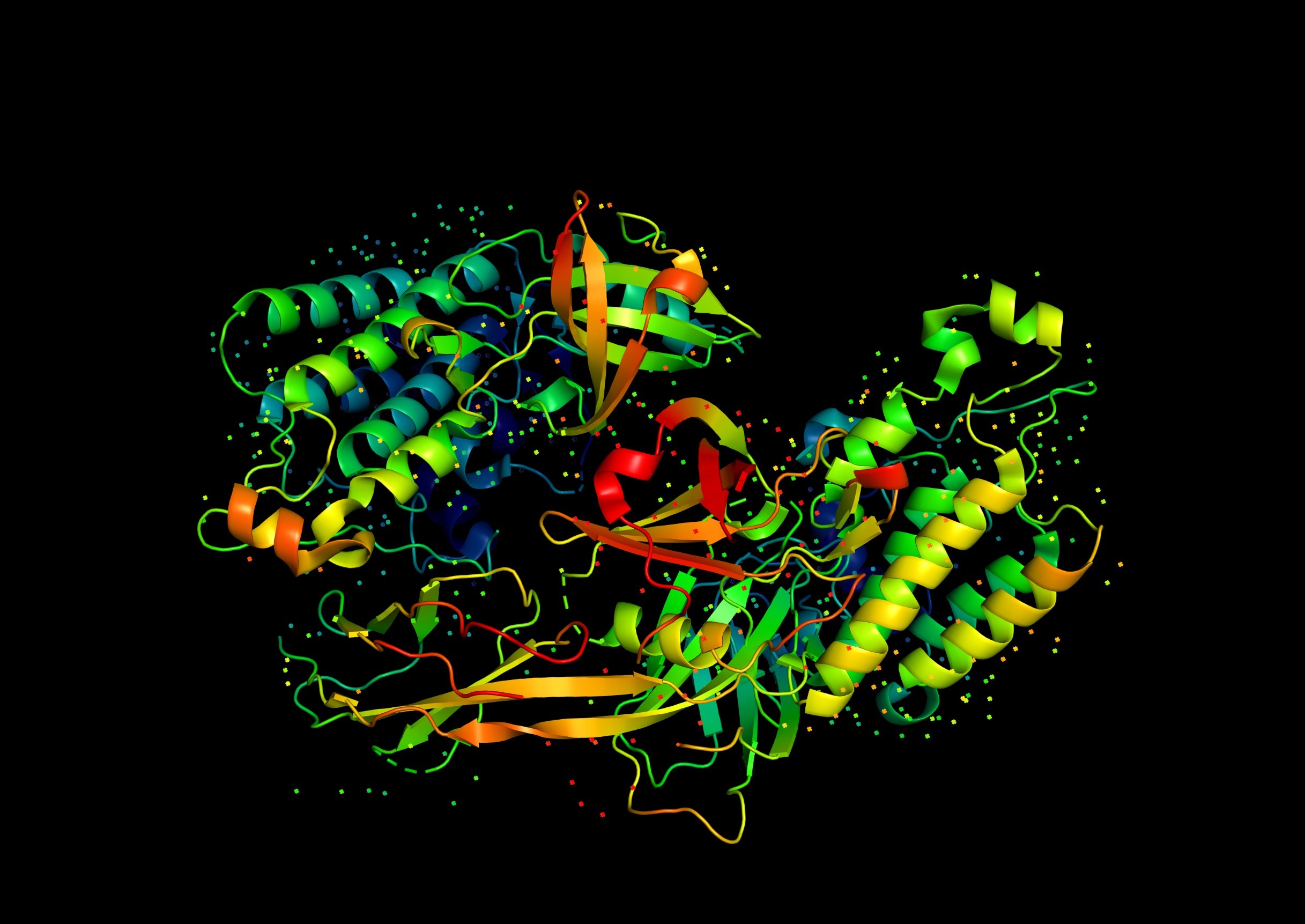
Structure of the Rop Protein
The Rop protein consists of two identical subunits, each containing 63 amino acids. These subunits dimerize to form a four-helix bundle, a common structural motif in proteins. The helical structure of Rop is characterized by two antiparallel α-helices from each monomer that interact with each other to create a stable, symmetrical four-helix bundle.
Key Structural Features
- Helix-Turn-Helix Motif: The protein’s dimerization interface involves a helix-turn-helix motif, which is essential for the proper alignment and interaction of the helices.
- Hydrophobic Core: The stability of the Rop dimer is maintained by hydrophobic interactions within the core of the four-helix bundle.
- Amphipathic Helices: The helices of Rop are amphipathic, meaning they have both hydrophobic and hydrophilic regions, facilitating proper folding and dimerization.
Function of the Rop Protein
The primary function of the Rop protein is to regulate the replication of plasmids by modulating the formation of RNA secondary structures that are critical for plasmid copy number control.
Mechanism of Action
- RNA II and RNA I Interaction: Plasmid replication initiation involves the synthesis of a primer RNA, called RNA II. Another RNA molecule, RNA I, acts as an antisense regulator, binding to RNA II and preventing it from serving as a primer for DNA replication.
- Rop Protein Mediation: The Rop protein enhances the binding of RNA I to RNA II, facilitating the formation of a stable RNA-RNA duplex. This interaction prevents RNA II from adopting the structure necessary for priming replication, thereby reducing plasmid replication initiation.
- Copy Number Control: By stabilizing the RNA I-RNA II interaction, the Rop protein helps maintain a low copy number of plasmids within the bacterial cell, ensuring that the plasmid does not over-replicate, which could be detrimental to the host cell.
Biological Significance
The regulation of plasmid copy number by the Rop protein is vital for several reasons:
- Genetic Stability: Maintaining an appropriate number of plasmid copies ensures genetic stability and prevents metabolic burden on the bacterial cell.
- Resource Allocation: Proper plasmid regulation allows efficient use of cellular resources, as excessive plasmid replication can deplete nucleotides and other essential molecules.
- Biotechnological Applications: Understanding the Rop protein’s role in plasmid replication has implications for biotechnology, where plasmids are used as vectors for gene cloning, protein expression, and genetic engineering.
Research and Applications
The Rop protein has been extensively studied as a model system for understanding protein folding, stability, and interactions. Its relatively simple structure makes it an ideal candidate for biophysical and structural studies.
Protein Engineering
Researchers have utilized the Rop protein to explore principles of protein design and engineering. Mutagenesis studies on Rop have provided insights into:
- Protein Stability: Mutations that affect the hydrophobic core or helix interactions have been used to study the factors contributing to protein stability.
- Folding Pathways: The folding mechanism of the Rop protein serves as a model for understanding how proteins achieve their native conformation.
- Functional Modulation: By altering specific residues, scientists have been able to modify the regulatory function of Rop, providing a deeper understanding of protein-RNA interactions.
Synthetic Biology
In synthetic biology, the principles derived from studying the Rop protein are applied to design synthetic regulatory circuits. These circuits can control plasmid copy number, gene expression, and other cellular processes with high precision.
The Rop protein is a fundamental component in the regulation of plasmid replication in bacteria, ensuring genetic stability and efficient use of cellular resources. Its well-defined structure and pivotal role in RNA-mediated regulation make it an important model for studying protein interactions, stability, and folding. Insights gained from research on the Rop protein not only enhance our understanding of bacterial genetics but also contribute to advancements in biotechnology and synthetic biology.
Leave a Reply